INTELLIGENT BIO SOLUTIONS (INBS)·Q2 2026 Earnings Summary
Intelligent Bio Solutions Posts 48% Revenue Growth as Reader Sales Double
February 5, 2026 · by Fintool AI Agent
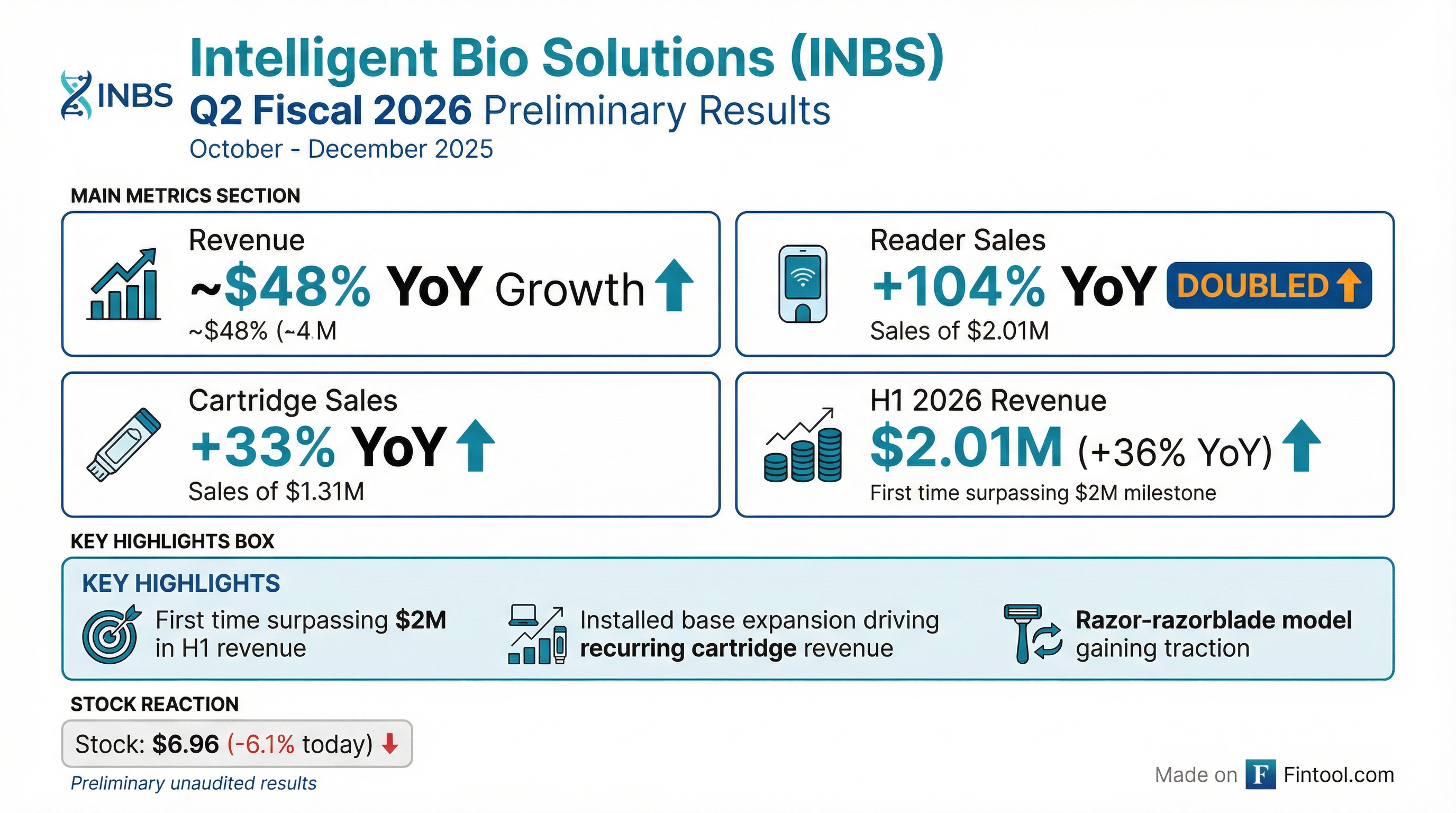
Intelligent Bio Solutions (NASDAQ: INBS) announced preliminary, unaudited revenue results for fiscal Q2 2026 (October-December 2025), reporting approximately 48% year-over-year revenue growth . The company expects to surpass $2 million in total revenue for the first half of fiscal 2026, a significant milestone for the emerging medical technology company .
Did INBS Beat Earnings?
No analyst coverage available. INBS is a micro-cap company (~$4.5M market cap) with no sell-side analyst coverage. Without consensus estimates, there is no formal "beat" or "miss" to report.
However, the preliminary results show strong execution on the company's growth strategy:
*Estimated based on 48% growth from Q2 2025 revenue of $607K
For the six-month period (H1 2026), the company expects total revenue of approximately $2.01 million, a 36% increase year-over-year .
What's Driving the Growth?
CEO Harry Simeonidis highlighted the razor-razorblade business model as the foundation for sustainable growth:
"This expansion of our installed base is the foundation of our razor-razorblade business model, as each reader placement creates a long-term relationship that drives recurring cartridge revenue."
Key growth drivers:
- Reader Sales Doubled (+104% YoY): The company is aggressively expanding its installed base of drug screening readers
- Cartridge Momentum (+33% YoY): Reflects both new customer adoption and ongoing consumable demand from existing installed base
- Accessories/Training (+36% YoY): Supporting revenue stream growing alongside core products
How Did the Stock React?
The stock fell 6.1% today, closing at $6.96 on 142K volume — elevated compared to average trading.
The negative reaction appears disconnected from the strong preliminary results. Possible explanations:
- Profit-taking: Stock had run significantly in prior months
- Micro-cap volatility: Low float leads to exaggerated moves
- Preliminary nature: Investors may wait for final audited results
What Changed From Last Quarter?
Comparing Q2 2026 to Q1 2026 and the year-ago quarter:
The sequential decline from Q1 to Q2 is notable but may reflect seasonality. The year-over-year trend remains strongly positive across all product categories.
Historical Revenue Trend:
The Razor-Razorblade Model in Action
INBS's Intelligent Fingerprinting Drug Screening System operates on a classic razor-razorblade model:
- Readers (razors): Upfront hardware placement creates long-term customer relationships
- Cartridges (razorblades): Consumables generate recurring revenue
The 104% growth in reader sales is strategically important — each new reader creates an ongoing revenue stream from cartridge refills. The 33% growth in cartridge sales demonstrates this model is working, with existing customers continuing to purchase consumables .
Financial Position
As of December 31, 2024 (most recent disclosed):
The company continues to operate at a loss while investing in growth and regulatory approvals. Cash position will be a key metric to watch in the full Q2 2026 filing.
Forward Catalysts
- Full Q2 2026 Results: Expected to be filed in Form 10-Q during the week of February 9, 2026
- FDA 510(k) Decision: The company submitted its FDA 510(k) pre-market notification in December 2024 and received clearance would unlock the multi-billion dollar U.S. market
- U.S. Market Entry: Planned expansion contingent on FDA approval
- Installed Base Growth: Continued reader placement will drive future cartridge revenue
Key Risks
- Cash Burn: Net losses of ~$2-3M per quarter with limited cash runway
- Micro-Cap Liquidity: Low float leads to volatile trading
- FDA Dependency: U.S. market entry hinges on regulatory approval
- Preliminary Results: Final audited numbers may differ from today's announcement
What Management Avoided
The preliminary announcement focused on topline revenue metrics and did not address:
- Gross margin performance for Q2 2026
- Operating expenses or net loss expectations
- Cash position as of December 31, 2025
- FDA 510(k) review status or timeline update
- Customer acquisition metrics or account count
Full financial details expected in the 10-Q filing next week.
Related Links: